A few semesters back, a student sent an email to one of my faculty explaining that “I’m really good at algebra, it’s just that chemistry algebra that gets me.” I had always interpreted that statement as a frustration with the problem-solving nature of freshman chemistry. Maybe the student was good at algebra but just couldn’t handle the word problems or logic that comes with chemistry.
This semester has made me rethink that notion. I work at a small state university with a low bar for admission. The general chemistry classes are populated with all majors, some college ready and some not. For most of the students in my classes, general chemistry I is a check box to be ticked. That being said, I’ve noticed some alarming trends.
This material is posted on the Flipped Chemistry website.
A few years ago our department tightened up our math prerequisites for admission into general chemistry I. We only admit students if they score high enough on the ACT or SAT to be admitted to the precalculus class or have passed college algebra with a “C” or better. The remarks below concern students who have met this requirement.
I recently tutored a student who had a poor score on the second hour exam. One of the problems was a titration with sulfuric acid. I was trying to explain that sulfuric acid was the acid made from the sulfate anion. After a while, we got to this point: how many protons must one add to the sulfate ion to produce a neutral species. The student drew a blank, so I thought it would be helpful to write out the equation:
X + -2 = 0
It turned out that the solution was not transparent to the student. Their approach was to guess until I told them the answer was correct. I cut them off after they guessed the correct answer on guess number four so I don’t know if, left alone, if they would have eventually realized that “2” would have done the trick.
Same student, different problem. The exam question asked for the oxidation number of carbon in the carbonate ion. The carbonate ion was written as CO32-. After a bit, we got to the point of realizing that the oxidation number of the carbon had to add to the combined oxidation number of the oxygens to give the charge on the ion. This drew a blank look. So I wrote on the board:
X + -6 = -2
No luck here, either. The student’s first guess was -8. It did get better from there, but the underlying process was “guess until Dr. Osterhout says it’s right.”
To do a quick assessment of the class’s math skills I gave a first day quiz this semester that included some math problems to be worked without a calculator (you can see the quiz and the results on the worksheets page of this website. Here is my favorite:
a = b/c, solve for c.
In the Fall of 2016, 59% of the students correctly solved the problem. Sadly, this Fall the number was 47%. It does not bode well for your future in chemistry if you are in the 53% that can’t do this little bit of algebra.
Here is one designed to see if they know anything about manipulating numbers in scientific notion. Remember, they could not use a calculator for this one.
3.0 x 104 x 4.0 x 103 =
This garnered 75% and 66% in Fall of 2016 and 2017 respectively. I was a little surprised that the results were so favorable. But then there was this:
2.0×106 x 4.0×104 / 8.0×10-3
That one was 18% in 2016 and 14% in 2017. The students don’t fare much better if they use their calculators. Even at the end of the semester, I have students who still use the 10x key on their calculator to enter numbers in scientific notion. I also find that students have blithely ignored my exhortations to use the SCI option on their calculators and read, for instance, 6.43 x 10-8 instead of 0.000000064 on their displays.
Recently we were doing calorimetry. In one variety of problems the students are asked to find the final temperature after, say, hot water is added to an iron pot. The equations encountered were of the following variety.
g x cs x (Tf – Ti) = -g x cs x (Tf – Ti)
The students had to solve for Tf. They gathered up the appropriate numbers and happily plugged and chugged until:
1021(Tf – 25) = -3138(Tf – 95)
(I left out the units, because I’m illustrating the math for you.) At this point four out of eight students in the tutorial session were unable to distribute the numbers into the parentheses. I fear that it is much worse in the class in general, because the only students coming to the tutorial had a least a marginal grasp on the material. The ones that really needed to come, didn’t. Once we got over that hurdle, we arrived at:
1021Tf – 25530 = -3138Tf + 298000
Here again, half of the students couldn’t proceed.
My experience in the last few years implies that it isn’t necessarily “that chemistry algebra,” sometimes it is just plain old algebra (and maybe just some sixth grade arithmetic). Keep in mind that all of these students had either passed college algebra or had a 600 or greater on the math portion of the SAT. So, what’s a mother to do?
Currently our general chemistry professors build math and calculator exercises into their homework and class materials. Clearly it is not enough. Our department has discussed developing a chemistry tutorial that would meet one hour a week to do remedial math and chemistry. We have experienced push back from the administration because they fear this would diminish our enrollment capacity ($). However, it would likely improve retention ($$). Any ideas?
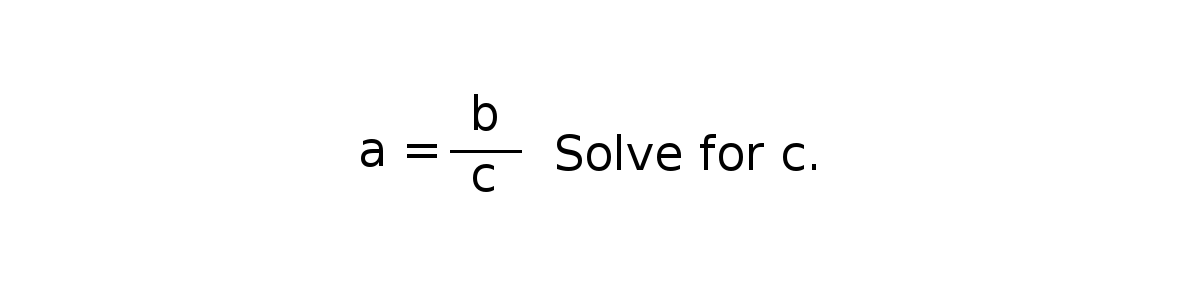
Hi John,
I teach at an open enrollment State College (which actually enrolls 90%+ A.S. and A.A. students). “Developmental” or “College Prep” remedial courses are no longer required in my state, therefore enrollment in development courses is strictly voluntary and we know that students do not typically pursue “voluntary” coursework (requiring hours of work and additional tuition). This means that many more students come to chemistry classes with the same obstructions to understanding that you illustrate. The text “Calculations in Chemistry” by Dahm and Nelson (published by Norton) was written to address these issues of weak problem solving skills. Unfortunately teaching any course is a zero sum game – there is never enough time to teach “Chemistry” as well as “Problem Solving” for chemistry. I am still searching for a solution to this problem. Good luck on your search!